Ammonium chloride
| Ammonium chloride | |
|---|---|
 |
|
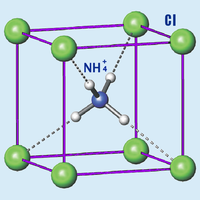 |
|
|
Ammonium chloride
|
|
|
Other names
Sal ammoniac
|
|
| Identifiers | |
| CAS number | 12125-02-9 |
| ChemSpider | 23807 |
| EC number | 235-186-4 |
| RTECS number | BP4550000 |
|
SMILES
[Cl-].[NH4+]
|
|
|
InChI
InChI=1/ClH.H3N/h1H;1H3
Key: NLXLAEXVIDQMFP-UHFFFAOYAI |
|
| Properties | |
| Molecular formula | NH4Cl |
| Molar mass | 53.491 g/mol |
| Appearance | White solid hygroscopic |
| Odor | odorless |
| Density | 1.5274 g/cm3 |
| Melting point |
338 °C (decomposes) |
| Solubility in water | 29.7 g/100 mL (0 °C) 37.2 g/100 mL (20 °C) 77.3 g/100 mL (100 °C) |
| Solubility in alcohol | 0.6 g/100 mL (19 °C) |
| Acidity (pKa) | 9.245 |
| Refractive index (nD) | 1.642 |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−314.55 kJ/mol[1] |
| Standard molar entropy S |
94.85 J K−1 mol−1 [1] |
| Hazards | |
| MSDS | ICSC 1051 |
| EU Index | 017-014-00-8 |
| EU classification | Harmful (Xn) Irritant (Xi) |
| R-phrases | R22, R36 |
| S-phrases | (S2), S22 |
| NFPA 704 |
 0
1
0
|
| Flash point | Non-flammable |
| LD50 | 1650 mg/kg, oral (rat) |
| Related compounds | |
| Other anions | Ammonium fluoride Ammonium bromide Ammonium iodide |
| Other cations | Sodium chloride Potassium chloride Hydroxylamonium chloride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Ammonium chloride NH4Cl (also Sal ammoniac, salmiac, nushadir salt, sal armagnac, sal armoniac, salt armoniack) is, in its pure form, a clear white water-soluble crystalline salt of ammonia. The aqueous ammonium chloride solution is mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride. The mineral is especially common on burning coal dumps (formed by condensation of coal-derived gases), but also on some volcanoes.
Contents |
Sources
The substance occurs naturally in volcanic regions, forming on volcanic rocks near fume-releasing vents. The crystals deposit directly from the gaseous state, and tend to be short-lived, as they dissolve easily in water. It is a by-product of the Solvay process used to produce sodium carbonate.[2]
Ammonium chloride is prepared commercially by reacting ammonia (NH3) with hydrogen chloride (HCl). As these chemicals are corrosive, this process has to be performed in vessels lined with nonreactive materials (e.g. glass, enamel, lead, or PVC).[2]
- NH3 + HCl → NH4Cl
This reaction can occur if poorly sealed bottles of household ammonia (ammonium hydroxide) and hydrochloric acid are stored in close proximity, leading to crystals forming around the openings of the bottles (mostly appear on those leaking more slowly).
Reactions
Ammonium chloride appears to sublime but this process actually involves decomposition into ammonia and hydrogen chloride gas.[2]
- NH4Cl → NH3 + HCl
Ammonium chloride may be reacted with a hydroxide base, e.g. sodium hydroxide, to release ammonia gas:
- NH4Cl + NaOH → NH3 + NaCl + H2O
If test tubes of ammonia solution and hydrochloric acid are brought close together, a smoke composed of microcrystals of ammonium chloride will slowly rise out of the tube.
Applications

Chemistry and Physics
Ammonium chloride dissolved in water becomes an acid. Ammonium chloride is used to produce low temperatures in cooling baths. For example, the zero point of Fahrenheit temperature scale is determined by placing the thermometer in a mixture of ice, water, and ammonium chloride. Ammonium chloride solutions with ammonia are used as buffer solutions.
Biology and Agriculture
In biological applications ammonium chloride acts as a nitrogen source and is used in fertilizers, as a feed supplement for cattle and as an ingredient in nutritive media for yeast microbiological organisms.
Pyrotechnics
Ammonium chloride is an ingredient in fireworks and safety and contact explosives.
Textile and Leather
Ammonium chloride is used in the textile and leather industry in dyeing, tanning textile printing and to luster cotton.
Metalwork
Ammonium chloride is used as a flux in preparing metals to be tin coated, galvanized or soldered. It works as a flux by cleaning the surface of workpieces by reacting with the metal oxides at the surface to form a volatile metal chloride. It is sold in blocks at hardware stores for use in cleaning the tip of a soldering iron and can also be included in solder as flux.
Medicine
Ammonium chloride is used as an expectorant in cough medicine. Its expectorant action is caused by irritative action on the bronchial mucosa. This causes the production of excess respiratory tract fluid which presumably is easier to cough up.
Ammonium salts are an irritant to the gastric mucosa and may induce nausea and vomiting.
Ammonium chloride is used as a systemic acidifying agent in treatment of severe metabolic alkalosis, in oral acid loading test to diagnose distal renal tubular acidosis, to maintain the urine at an acid pH in the treatment of some urinary-tract disorders
Ammonium Chloride is also used as a diuretic in forced acid diuresis.
Food
In several countries ammonium chloride is known as sal ammoniac and used as food additive. The E number for ammonium chloride used as a food additive is E510.
Sal ammoniac is used to spice up dark sweets called salty liquorice and in the flavouring Salmiakki Koskenkorva for vodkas.
Sal ammoniac is also used in baking to give cookies a very crisp texture.
Other Applications
Ammonium chloride is used in a ~5% aqueous solution to work on oil wells with clay swelling problems. It is also used as electrolyte in Zinc–carbon batteries. Other uses include in hair shampoo, in the glue that bonds plywood, in cleaning products.
References
- ↑ 1.0 1.1 Solid state data from Ammonium chloride in Linstrom, P.J.; Mallard, W.G. (eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology, Gaithersburg MD. http://webbook.nist.gov (retrieved 2008-10-22)
- ↑ 2.0 2.1 2.2 Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0123526515, p. 614
|
|||||||||||||||||||||||||||||||||||||||||||